
Wai Leong TAM
Cancer progression is orchestrated by complex alterations in cellular behaviors that enable the survival of, and colonization by, cancer cells in the human body. Disturbances in cell states underlie disease progression and clinical outcomes. Underpinning the control of cancer cell states are metabolic reprogramming events, i.e., why are the nutritional needs of cancer cells unique or different? Many fundamental cancer pathways are now known to impinge on cell metabolism, and strategies focusing on disrupting specific metabolic processes to control disease progression have gained considerable interests. Leveraging on functional genomic approaches, and in close collaboration with clinician partners, Dr Wai Leong Tam’s group seeks to gain insights into the precise control of cancer cell states, and specific targeting of metabolic pathways, for the development of selective therapeutics against cancer resistance and metastasis.
tamwl[at]a-star.edu.sg
Principal Investigator, Cancer Science Institute of Singapore, NUS
Director, Graduate Affairs, Biomedical Research Council, A*STAR
Deputy Executive Director, Genome Institute of Singapore, A*STAR
Associate Director, Genome Institute of Singapore, A*STAR
Associate Professor (Adjunct), Department of Biochemistry, Yong Loo Lin School of Medicine, NUS
| 2023 – present | Singapore NRF Investigator, National Research Foundation, Singapore |
| 2015 – 2020 | Singapore NRF Fellow, National Research Foundation, Singapore |
| 2003 – 2007 | A*STAR Graduate Scholarship, A*STAR |
| 2003 – 2006 | President’s Graduate Fellowship, NUS |
| 2003 | Vice-Chancellor’s List, NUS |
| 2003 | Joanna Wong Gold Medal Award, University Scholars Programme, NUS |
| 2002 – 2003 | Frazer & Neave Book Prize, Faculty of Science, NUS |
| 2001 – 2003 | Raffles Prize, Faculty of Science, NUS |
| 2000 – 2003 | Dean’s List (every year), NUS |
While significant headways have been made in the development and deployment of therapeutics for some cancers, long-term disease-free responses for most cancers are rare. Drug development efforts have traditionally focused on well-studied kinases, hormones and receptors, resulting in a saturation of targets that already have multiple inhibitors. Adding to this challenge, tumor cells evolve over time, acquire bypass pathways, and gain resistance to multi-generations of drug classes, highlighting the limitations of such approaches. Thus, there is an unmet need to develop newer classes of therapeutic modalities that may be more durable, and well as more innovative ways to control cancer growth and dissemination through the precise control of cell states.
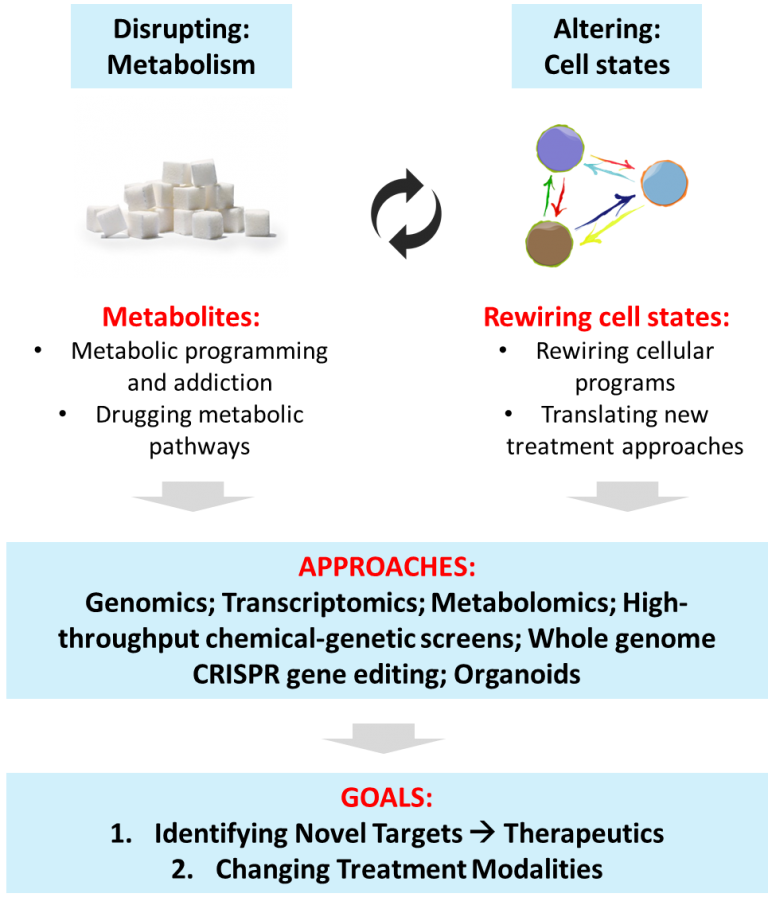
Cellular Metabolism: Dysregulated cellular metabolism is a more recent addition to the hallmarks of cancer. The revelation that tumor cells and their associated stromal cells have altered metabolic requirements suggests a way forward in targeting their metabolic states. Several of the most effective drugs that have been in use for many decades target cancer cell metabolism. In fact, the very first chemotherapeutic agent is an anti-metabolite (anti-folate), and it is still used as the standard-of-care more than fifty years since its discovery. This highlights the longevity of anti-metabolites and their immense potential as durable anti-cancer agents. Altered metabolism, such as the avid uptake and utilization of glucose and certain amino acids including serine, glycine and glutamine are well-described. These modes of metabolism appear critical for tumor proliferation in general. Owing to the diverse intratumoral subpopulations that include cancer stem cells and drug resistant cells, further investigations are needed to develop more specific anti-cancer metabolic strategies for durable clinical responses.
What are the metabolites produced and utilized by heterogeneous tumor cells that include CSCs, resistant cells, and disseminated metastatic cells? Why are they uniquely important? How do we exploit the metabolic liabilities of cancer as therapeutic targets?
Cellular Energetics: Dysregulated cellular energetics (i.e. cancer metabolism) is a more recent addition to the hallmarks of cancer. The revelation that tumor cells and their associated stromal cells have altered metabolic requirements suggests a way forward in targeting their metabolic states. Several of the most effective drugs that have been in use for many decades target cancer cell metabolism. In fact, the very first chemotherapeutic agent is an anti-metabolite (anti-folate), and it is still used as the standard-of-care more than fifty years since its discovery. This highlights the longevity of anti-metabolites and their immense potential as durable anti-cancer agents. Altered metabolism, such as the avid uptake and utilization of glucose and certain amino acids including serine, glycine and glutamine are well-described. These modes of metabolism appears critical for tumor proliferation in general. Owing to the diverse intratumoral subpopulations that include cancer stem cells and drug resistant cells, further investigations are needed to develop more specific anti-cancer metabolic strategies for durable clinical responses.
What are the metabolites produced and utilized by heterogeneous tumor cells that include CSCs, resistant cells, and disseminated metastatic cells? Why are they uniquely important? How do we exploit the metabolic liabilities of cancer as therapeutic targets?
Cell State Transitions: Cellular heterogeneity at the genomic and transcriptomic levels is a hallmark of tumors. The heterogeneous nature of tumors may be driven by evolution of distinct clonal cell population as a result of selection or through inherent phenotypic plasticity. Cellular plasticity may be enabled by cell state transitions that is regulated through gene expression programs with far-reaching consequences. Cell state transitions include the epithelial vs mesenchymal, stemness vs dedifferentiation, therapy sensitive vs resistance, and local tumor vs metastases. Understanding cellular pathways and programs governing phenotypic plasticity will result in more precise control of cell states and cell fates, thereby providing new opportunities to alter the course of disease progression.
How do we engineer vulnerabilities into specific populations of aggressive cancer cells that will cause them to gain susceptibility to therapy? Can we rewire stemness and differentiation programs in cancer cells?
Tumor Microenvironment Exchanges: Cancer cells utilize nutrients in a cell-autonomous manner, but cell-extrinsic factors arising from the tumor microenvironment have been recently observed. The notion that stromal and tumor cells engage in metabolic exchange is nascent. Understanding the extent and nature of such metabolic exchanges between tumor and microenvironment cells can inform on the use therapeutics to target metabolic vulnerabilities, including their impact on the emerging paradigm of immune-oncometabolism. Harnessing whole-metabolome functional chemical-genetic screens, coupled to our unique resource of patient-derived cancer+stromal cells and our curated metabolic drug libraries, we will interrogate their metabolic exchange and induced dependencies. Cancer-stromal co-culture organoid systems will be adopted to understand how stromal cells (cancer-associated fibroblasts, macrophages and T-lymphocytes) program metabolic addiction in cancer cells. Conversely, how cancer cells secrete metabolites to nourish and sustain a pro-tumor microenvironment through influencing the metabolism of immune cells will also be examined.




















