
Motomi OSATO
We are investigating the mechanism behind the development of leukaemia by analyses of a key leukaemia gene, RUNX1. Through these studies, we aim to develop novel diagnostic and therapeutic methods. To achieve this, we use a range of experimental platforms, such as mouse models as well as standard biological and chemical techniques. Our best known research accomplishment is the detection of RUNX1 point mutation in acute myeloid leukaemia. This genetic change is included in the World Health Organization (WHO) leukaemia classification and being examined globally whenever a leukaemia diagnosis is made.
csimo[at]nus.edu.sg
Principal Investigator, Cancer Science Institute of Singapore, National University of Singapore
Research Associate Professor, Department of Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore
Professor (visiting), International Research Center for Medical Sciences, Kumamoto University, Japan
Leukemia research towards novel therapeutics
We have been interrogating the mechanistic basis for leukemogenesis through the analyses of a key leukemia gene, RUNX1, aiming for the development of novel diagnostic and therapeutic methods (Figure 1). Treatment of RUNX leukemia has a dismal outcome and novel therapeutic approaches are needed. Our experimental platforms inlcude a mouse system (knockout or transgenic mice), and molecular and cellular analyses on clinical samples. Using such strategy, we have identified many diagnostic markers and treatment methods.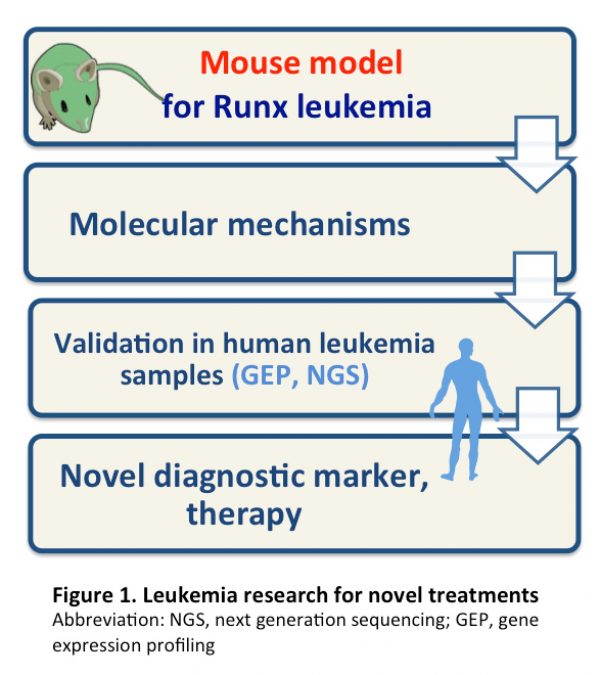
Identification of cis-regulatory elements associated with human diseases
Human genome consists of 1.5% of coding and 98.5% of non-coding regions. Approximately 40% of disease-related genetic changes are expected to be located within the non-coding region, particularly in cis-regulatory elements (enhancer, silencer, locus control region, and insulator) which govern expression of genes. Genetic alterations in non-coding (intronic and intergenic) regions in RUNX loci have also been suspected to be the underlying mechanism for multiple human diseases; however, cis-elements for Runx family genes remain largely unknown. Employing our own unique strategy as shown in Figure 2, we have identified multiple cis-regulatory elements. Single nucleotide polymorphisms (SNPs) within these elements would serve as predictive risk factors for human diseases, whereas pharmaceutical modulation of the elements would lead to novel therapeutic directions.









