Michael A. Longo1 ∙ Syed Moiz Ahmed2,10 ∙ Yue Chen3,10 ∙ Chi-Lin Tsai1,10 ∙ Sarita Namjoshi1 ∙ Runze Shen1 ∙ Zamal Ahmed1 ∙ Xiaoyan Wang1 ∙ Rajika L. Perera4 ∙ Andy Arvai5 ∙ Miyoung Lee6 ∙ Li Ren Kong2,6 ∙ Wilfried Engl7 ∙ Woei Shyuan Ng7 ∙ Ziqing Winston Zhao7,8 ∙ Ashok R. Venkitaraman2,6,9∙ John A. Tainer1,3 ∙ Katharina Schlacher3,11
- Department of Molecular & Cellular Oncology, UT MD Anderson Cancer Center, Houston, TX 77030, USA
- The Cancer Science Institute of Singapore, National University of Singapore, Singapore 117599, Singapore
- Department of Cancer Biology, UT MD Anderson Cancer Center, Houston, TX 77054, USA
- Poseidon Laboratory, Pasadena, CA 91107, USA
- The Department of Integrative Structural & Computational Biology, The Scripps Research Institute, La Jolla, CA 92037, USA
- Medical Research Council Cancer Unit, University of Cambridge, Hills Road, Cambridge CB2 0XZ, UK
- Department of Chemistry and Centre for BioImaging Sciences, National University of Singapore, Singapore 117543, Singapore
- Mechanobiology Institute, National University of Singapore, Singapore 117411, Singapore
- Institute of Molecular & Cell Biology, Agency for Science, Technology and Research (A∗STAR), Singapore 138673, Singapore
- These authors contributed equally
- Lead contact
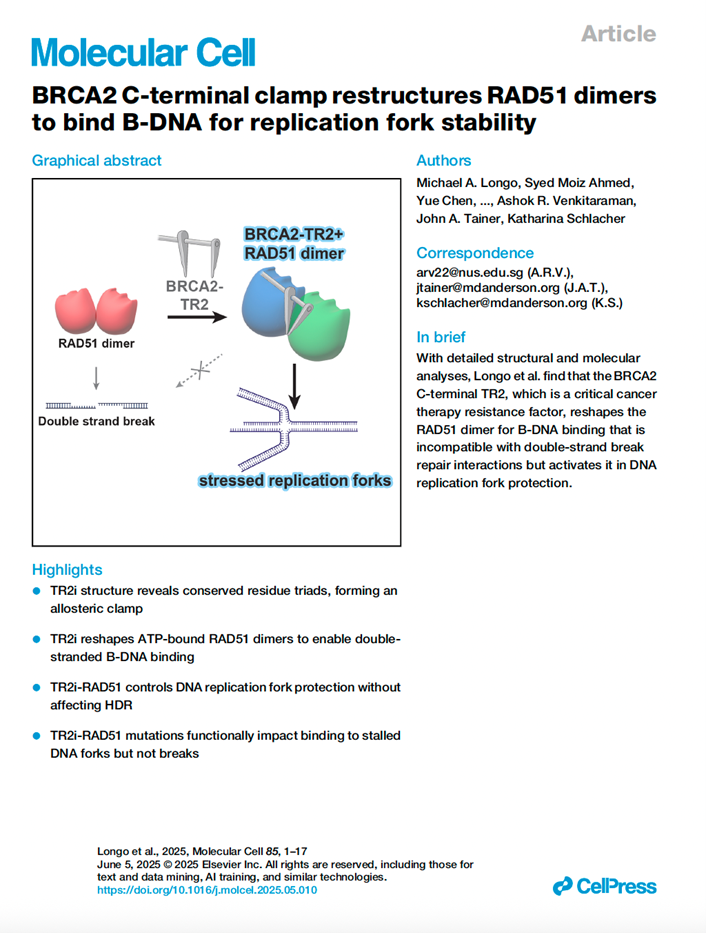
Tumor suppressor protein breast cancer susceptibility protein 2 (BRCA2) acts with RAD51 in replication fork protection (FP) and homology-directed DNA-break repair (HDR). Critical for cancer etiology and therapy resistance, the BRCA2 C terminus was thought to stabilize recombinogenic RAD51 after the assembly of ATP-extended RAD51 filaments on single-stranded DNA (ssDNA). Here, the detailed crystal structure of the human BRCA2 C-terminal interaction domain (TR2 interface [TR2i]) complexed with ATP-bound RAD51 prior to DNA binding instead reveals TR2i unexpectedly induces a unique ATP-RAD51 dimer conformation that accommodates nucleation onto double-stranded B-DNA unsuited for HDR initiation. Structural, biochemical, and molecular results with interface-guided mutations uncover TR2i’s FP mechanism. Proline-driven secondary structure stabilizes residue triads and spans the RAD51 dimer, engaging pivotal interactions of RAD51 M210 and BRCA2 S3291/P3292, the cyclin-dependent kinase (CDK) phosphorylation site that toggles between FP during S phase and HDR in G2. TR2i evidently acts as an allosteric clamp, switching RAD51 from ssDNA to double-stranded and B-DNA binding, enforcing FP over HDR, challenging the current BRCA2-RAD51 dogma. DOI: 10.1016/j.molcel.2025.05.010