Peter YEOW
Cell division is essential for tissue growth and repair—but when this process goes unchecked, it can lead to cancer. To sustain rapid growth, cancer cells often rewire the machinery that controls division. These changes can create unexpected weak points. Our lab studies these trade-offs to uncover and target vulnerabilities unique to cancer.
We also investigate TRIM proteins, regulators of large assemblies involved in cell division, immunity, and protein quality control. These systems are often disrupted in cancer. By understanding how TRIMs function, we aim to restore cellular balance or exploit their breakdown for therapeutic benefit.
zyyeow[at]nus.edu.sg
Principal Investigator, Cancer Science Institute of Singapore, NUS
Assistant Professor, Department of Biochemistry, Yong Loo Lin School of Medicine, NUS
Joint Principal Scientist I, A*STAR Institute of Molecular and Cell Biology (A*STAR IMCB)
|
Year |
Award |
|
2025 |
Presidential Young Professorship, National University of Singapore |
|
2025 |
The Alfred Blalock Research Award, Johns Hopkins University School of Medicine |
|
2020 |
Graduate Student Prize Winner, Nuffield Department of Medicine, University of Oxford |
|
2016 |
A*STAR National Science Scholarship (PhD) |
|
2011 |
ASEAN Undergraduate Scholarship |
The Yeow Lab investigates how cancer cells differ from normal cells, with a focus on the mechanisms that govern mitotic cell division and the regulation of mesoscale assemblies.
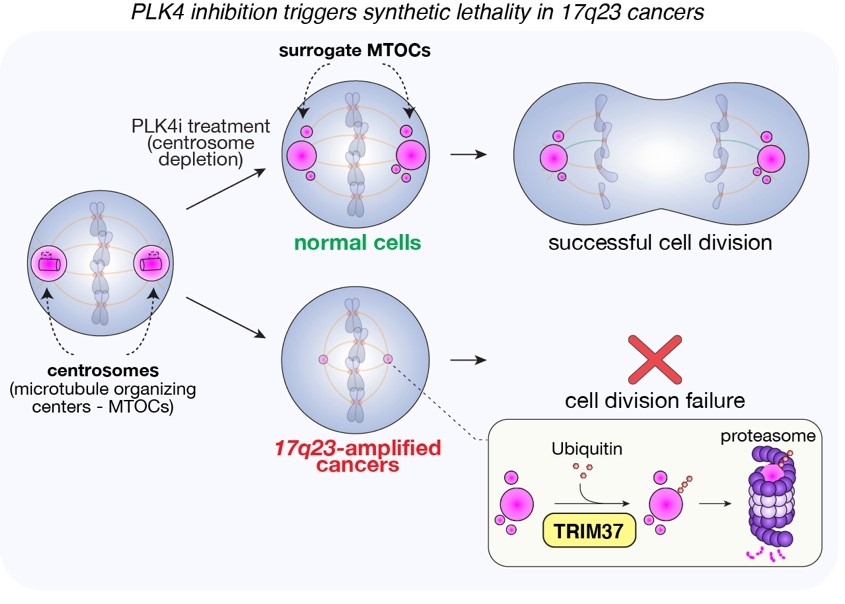
One branch of our research explores vulnerabilities in the mitotic machinery that cancer cells uniquely depend on. We showed that tumors carrying the 17q23 amplicon—present in ~10% of breast cancers and ~50% of neuroblastomas—are highly reliant on centrosomes, structures that organize the cell’s scaffolding during division. This synthetic lethal interaction identified TRIM37 as a key genetic driver and laid the foundation for ongoing clinical trials targeting the PLK4 kinase. We continue to search for additional weaknesses within the mitotic apparatus.
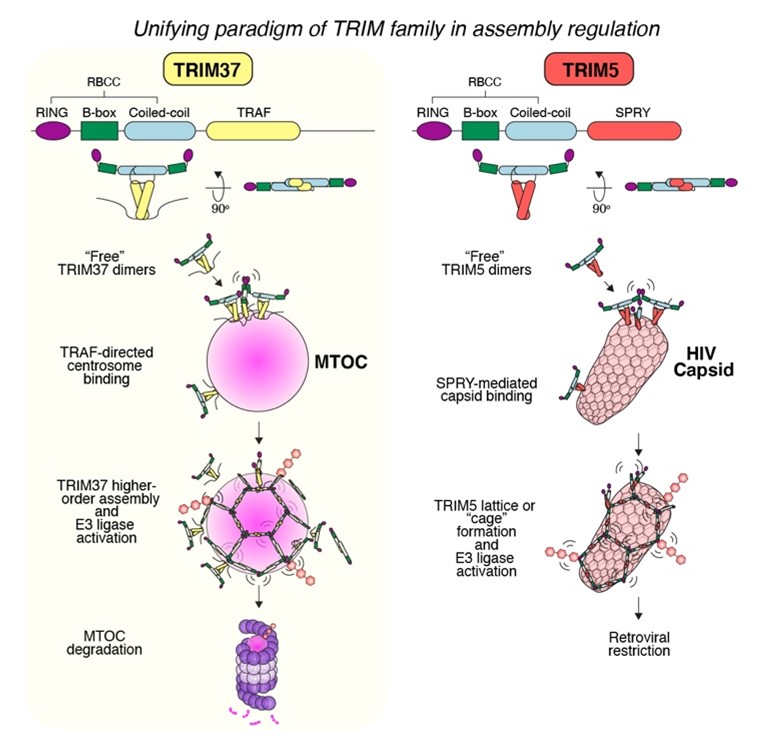
A second major focus centers on the TRIM family of E3 ubiquitin ligases. Our work revealed that TRIM37 can selectively dismantle aberrant microtubule-organizing centers—marking the first known case of a TRIM protein regulating an organelle. This mirrors TRIM5’s restriction of HIV capsids and suggests an evolutionarily conserved paradigm in which TRIM proteins surveil and regulate higher-order structures. We aim to define the ‘rules of engagement’ for TRIMs and unlock their therapeutic potential in cell division, immunity, and proteostasis.
Our goal is to develop precision cancer therapies grounded in mechanistic insight.